New Method May Improve Delivery of Targeted Gene Therapies, Study Reports
Written by |
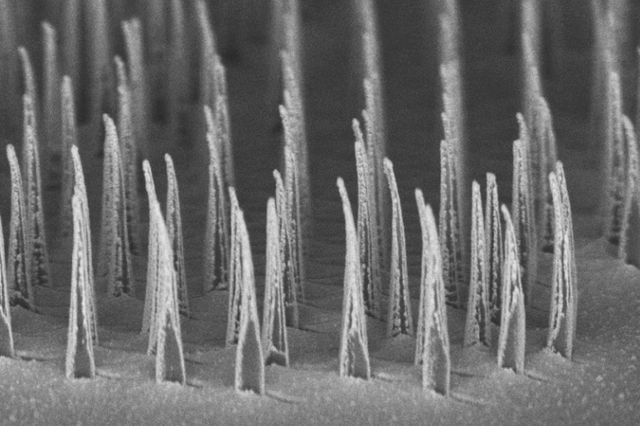
A delivery method using nanotubes with a spear-like shape may open new avenues for safer, faster, and more cost-effective gene therapies for several genetic diseases, including hemophilia, according to a study.
The study, “Precision-Guided Nanospears for Targeted and High-Throughput Intracellular Gene Delivery,” was recently published in the journal ACS Nano.
Current gene therapy approaches rely on modified viruses or potentially harmful chemicals to enter cells and deliver treatment. But these methods have some safety concerns, and can also be expensive and inefficient.
Developed by UCLA researchers, the new technology uses silicon, nickel, and gold to build tiny, biodegradable structures called “nanospears” to which a therapy or healthy gene can be attached. The nanospears are guided remotely with a magnetic field to ensure delivery of the correct gene to specific cells.
These nanospears can be inexpensively and efficiently produced, and have minimal impact on cell viability and metabolism due to their microscopic size — about 5,000 times smaller than the thickness of a human hair.
“The biggest barrier right now to getting either a gene therapy or an immunotherapy to patients is the processing time,” Steven Jonas, MD, PhD, a clinical fellow in the UCLA Broad Stem Cell Research Center Training Program and co-author of the study, said in a press release. “New methods to generate these therapies more quickly, effectively and safely are going to accelerate innovation in this research area and bring these therapies to patients sooner, and that’s the goal we all have.”
To test the method, researchers used a specific molecule that produces a green fluorescent protein inside cells. They showed that the nanospears could effectively enter cells and deliver the desired protein, which resulted in more than 80% of the cells exhibiting the green glow, of which more than 90% survived. These results demonstrate that the new method is not only efficient but also doesn’t have significant damaging effects on the cells.
These nanospears can also be adapted to currently available or investigative therapies, representing an effective option that can overcome the existing barriers of gene modification on a large scale, the researchers said.
“One of the amazing things about working at UCLA is that for each of the targeted diseases, we collaborate with leading clinicians who already have gene therapies in development,” said senior author Paul Weiss, PhD, UC presidential chair and a distinguished professor at UCLA. “They have the gene-editing cargo, model cells, animal models and patient cells in place so we are able to optimize our nanosystems on methods that are on the pathway to the clinic.”


