Roctavian (valoctocogene roxaparvovec-rvox) for hemophilia
What is Roctavian for hemophilia?
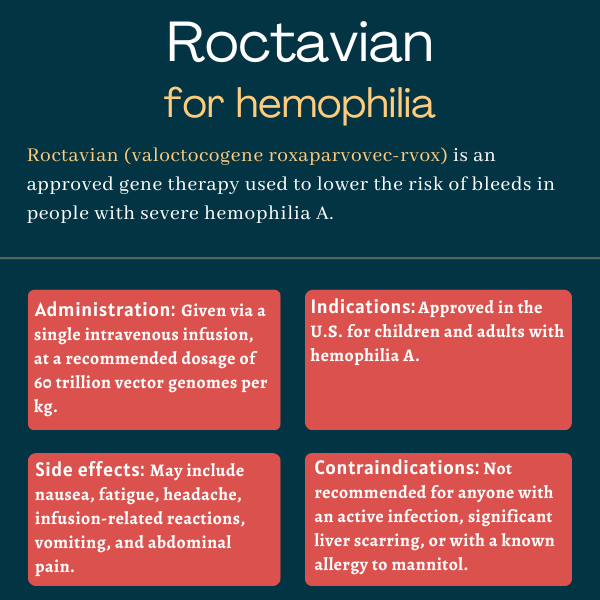
Roctavian (valoctocogene roxaparvovec-rvox) is an approved one-time gene therapy that’s intended to reduce the risk of bleeds in certain adults with severe hemophilia A.
Developed by BioMarin Pharmaceutical, the therapy is indicated for people who lack detectable antibodies against its viral carrier, which may render the treatment less effective or ineffective. It’s administered via a single infusion into the vein, or intravenously.
Therapy Snapshot
| Brand Name: | Roctavian |
| Chemical Name: | Valoctocogene roxaparvovec-rvox |
| Usage: | Gene therapy for people with hemophilia A; can reduce bleeds and the need for standard preventive treatment |
| Administration: | Intravenous infusion |
How does Roctavian work?
Hemophilia is a rare bleeding disorder caused by deficiencies in specific clotting proteins that mostly affects men.
Hemophilia A is marked by mutations in the F8 gene, which provides instructions for making a clotting protein called factor VIII (FVIII). Due to these mutations, the FVIII protein either doesn’t function correctly or not enough of it is made, leading to blood clotting abnormalities.
The standard treatment for hemophilia A is regular, lifelong infusions of FVIII replacement therapy to restore the levels of the missing clotting protein and prevent bleeds. About a third of patients will develop FVIII inhibitors, or neutralizing antibodies against FVIII, that can reduce the effectiveness of replacement therapies and raise the risk of severe bleeds.
Roctavian is a one-time gene therapy that uses a modified and harmless adeno-associated virus, called AAV5, to carry and deliver a shorter, but functional, version of the F8 gene to cells in the liver, the main producer of clotting factors.
The therapy, formerly known as Valrox or BMN 270, is expected to restore FVIII levels, thereby lowering the risk of bleeds, while reducing or potentially eliminating the need for routine prophylactic (preventive) replacement therapy.
Who can take Roctavian?
Roctavian was approved by the U.S. Food and Drug Administration (FDA) in 2023 to treat adults with severe hemophilia A without detectable antibodies against the therapy’s viral carrier. The presence of such antibodies must be assessed with an FDA-approved diagnostic test called AAV5 DetectCDx. The decision made Roctavian the first gene therapy to be approved for hemophilia A in the U.S.
Roctavian won conditional approval in Europe in 2022 for adults with severe hemophilia A without detectable anti-AAV5 antibodies and no previous history of inhibitors to standard replacement therapies. Its full approval is pending the receipt of additional data that confirms its clinical benefit in this indication.
Who should not take Roctavian?
Roctavian is not recommended for anyone with:
- an active infection, either acute (e.g., respiratory infection) or chronic (e.g., chronic hepatitis B)
- significant liver scarring or cirrhosis, a severe liver condition characterized by a scarred liver that is permanently damaged
- a known allergy to mannitol, one of its ingredients.
The therapy also shouldn’t be administered to patients with a history of or who currently have inhibitors to standard FVIII replacement therapies, nor to those with preexisting antibodies against AAV5. Patients with liver problems also should consider visiting a liver specialist to help determine if they’re eligible to receive Roctavian.
How is Roctavian administered?
Roctavian is available in glass vials containing 8 mL of a clear, colorless to pale yellow suspension containing 2×1013, or 20 trillion, vector genomes per milliliter. It’s administered via a single infusion into the bloodstream at a recommended dosage of 6×1013, or 60 trillion, vector genomes per kilogram of body weight (vg/kg).
It is administered with the help of an infusion pump at a rate of 1 mL per minute. The infusion rate may be increased every 30 minutes, up to a maximum rate of 4 mL per minute.
The therapy should be given under the supervision of a physician experienced in treating hemophilia and/or bleeding disorders, and in a setting where potential infusion-related reactions can be immediately addressed.
Roctavian in clinical trials
The approval of Roctavian was mainly supported by data from a Phase 3 clinical trial called GENEr8-1 (NCT03370913).
GENEr8-1 trial
The global GENEr8-1 study enrolled 134 men with severe hemophilia A (FVIII activity of 1% or lower of normal), at sites across 13 countries. All patients had been on standard preventive replacement therapy for at least six months, and none had a history of FVIII inhibitors or anti-AAV5 antibodies.
Participants received a single infusion of Roctavian at a dose of 6×1013 vg/kg, and were followed for at least 49 weeks. Preventive FVIII treatment was maintained through four weeks after infusion and FVIII was administered as needed thereafter.
The trial’s main goal was to assess the effect of treatment on FVIII activity over about one year post-dosing. Secondary measures included changes in bleeding rates and in the use of FVIII replacement therapy relative to the six months before receiving Roctavian. The data were available for 112 participants.
After a year, median FVIII activity levels were significantly increased to 23.9% of normal, results showed. More than a third of the participants (37.9%) had a median FVIII activity of 40% or higher — generally considered nonhemophilia levels — and 50% had FVIII activity levels ranging between 5-40%, usually indicating mild hemophilia.
Consistent with the increase in FVIII activity, mean annualized bleeding rates were significantly decreased by more than 80% (from 4.8 to 0.8 bleeds a year) and mean FVIII infusions dropped by about 99% (from 135.9 to two infusions a year) after one year. Most participants were bleed-free, starting five weeks after Roctavian treatment.
At two years post-treatment, bleeding rates were reduced by 77% and the use of FVIII declined by 98.2%, with 84% of patients being bleed-free and 95% remaining off prophylactic replacement therapy.
Three-year data demonstrated the median annualized rate of all bleeds and bleeds requiring treatment was zero, indicating that most participants didn’t have any bleeds.
Roctavian was associated with a more than 80% reduction in treated bleeds and a 52% drop in annualized bleeding rates after a median of three years. Mean annualized rates of spontaneous and joint bleeds were also reduced to less than one a year.
Also, FVIII usage was decreased by more than 95% after three years, with 92% of the patients not requiring prophylactic treatment. Median FVIII activity remained high, at 8.4% of normal.
BioMarin plans to assess Roctavian’s long-term effects in trial participants for up to 15 years in a long-term extension study.
Ongoing trials
A fully enrolled Phase 3b trial, called GENEr8-3 (NCT04323098) is evaluating the one-year efficacy of Roctavian when given alongside anti-inflammatory corticosteroids in 22 men with severe hemophilia A. The anti-inflammatory treatment is designed to prevent an immune response from developing antibodies against the therapy’s viral vector, which may lower its effectiveness.
The gene therapy also is being tested in up to 10 men with severe hemophilia A who have preexisting anti-AAV5 antibodies as part of a Phase 1/2 trial (NCT03520712). Participants will be followed for about five years after dosing.
Another Phase 1/2 study (NCT04684940) is assessing Roctavian’s five-year safety in up to 20 men with severe hemophilia A who have current or prior inhibitors against FVIII.
Common side effects of Roctavian
The most common side effects of Roctavian include:
- nausea
- fatigue
- headache
- infusion-related reactions
- vomiting
- abdominal pain.
The most common laboratory abnormalities associated with Roctavian include:
- higher than normal levels of liver enzymes, signaling liver toxicity
- higher than normal levels of the creatine phosphokinase enzyme, indicative of muscle injury or stress
- increased FVIII activity levels beyond the normal range.
Infusion-related reactions
Roctavian can cause infusion-related reactions, including allergic reactions and a severe form of allergic reaction known as anaphylaxis. These may include symptoms such as rash, sneezing, shortness of breath, nausea, diarrhea, low blood pressure, abnormally fast heartbeat, fever, and chills.
Patients should be monitored for possible signs of infusion-related reactions during and for at least three hours after receiving an infusion. If they occur, administration should be slowed or stopped and appropriate medication for infusion reactions should be considered. Roctavian infusion can be restarted at a lower administration rate after the infusion reaction has resolved. In case of anaphylaxis, the therapy should be discontinued altogether.
Liver toxicity
Roctavian may cause liver toxicity, presumably due to the immune system’s potential reactions against the therapy’s viral carrier, which is targeted to liver cells. This immune reaction may also reduce FVIII production and thereby limit Roctavian’s efficacy.
Markers of liver health should be evaluated before treatment (to determine a patient’s eligibility to receive Roctavian), then weekly for at least six months and less frequently thereafter.
Patients who develop higher than normal levels of certain liver enzymes, suggestive of liver damage, may need corticosteroid treatment, which should be started at a recommended dose of 60 mg, once daily for two weeks.
Liver enzymes should be monitored weekly, and as clinically indicated, during corticosteroid treatment and until their levels return to normal, after which corticosteroids should be tapered. Patients should also be monitored for adverse reactions secondary to corticosteroid therapy. If present, these reactions should be managed with appropriate medication.
Due to its potential to increase the levels of certain liver enzyme, alcohol shouldn’t be consumed for at least a year after a Roctavian infusion and its intake should be reduced thereafter. Physicians should also assess whether patients are taking medications, including herbal products and nutritional supplements, that can cause liver toxicity and consider using alternative medications.
Blood-clotting events
Because it works to increase the production of the FVIII clotting factor, Roctavian can result in higher than normal FVIII levels that may increase the risk for thrombosis, or blood-clotting events that disrupt blood flow.
Patients should be evaluated for their risk of thrombosis, including general cardiovascular risk factors, before and after receiving Roctavian. Preventive anti-blood-clotting medication should be considered for those with a higher thrombosis risk in relation to their FVIII activity levels.
Patients having signs or symptoms of thrombosis, such as chest pain, leg swelling and pain, trouble breathing, and skin changes, should seek immediate medical attention.
Monitoring laboratory tests
FVIII activity levels should be monitored in patients treated with Roctavian. If there is a reduction in FVIII activity levels or if bleeds aren’t well controlled after treatment with Roctavian, patients should also be assessed for the presence of FVIII inhibitors.
Liver cancer
While there were no reports of Roctavian-related cancer during clinical trials, the therapy carries a theoretical risk for hepatocellular carcinoma, the most common type of liver cancer. This is due to the potential ability of the therapy’s viral carrier to integrate the cells’ DNA, change a given DNA sequence, and cause cells to grow in an uncontrolled manner.
Patients with risk factors for hepatocellular carcinoma, such as hepatitis B or C infection, certain types of liver disease, and chronic alcohol consumption, should undergo regular liver ultrasound and specific blood tests for five years after treatment with Roctavian. If cancer develops, the patient’s physician should contact BioMarin at 1-866-906-6100 to obtain instructions on collecting patient samples for testing.
Use in pregnancy and breastfeeding
Roctavian is not intended for women, so there is no available data on its use during pregnancy or breastfeeding. There are also no animal studies evaluating its potential effects in these situations.
However, clinical and preclinical data showed that Roctavian’s delivered gene and/or viral carrier can be detected in semen and testes after treatment. As such, men receiving Roctavian and their female partners are recommended to use an effective form of contraception for six months after a Roctavian infusion. During this period, Roctavian-treated men should not donate semen.
Hemophilia News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Battling self-doubt as a woman with bleeding disorders
- Extended hemophilia half-life products aid care, but raise costs
- I’m encouraged to see my son take initiative in solving a problem
- Why asking for and accepting help is important for parents
- What I’ve learned about obesity, joint pain, and hemophilia bleeds
Related Articles

 Fact-checked by
Fact-checked by