What you need to know about gene therapy and hemophilia B
Last updated March 14, 2025, by Lindsey Shapiro, PhD

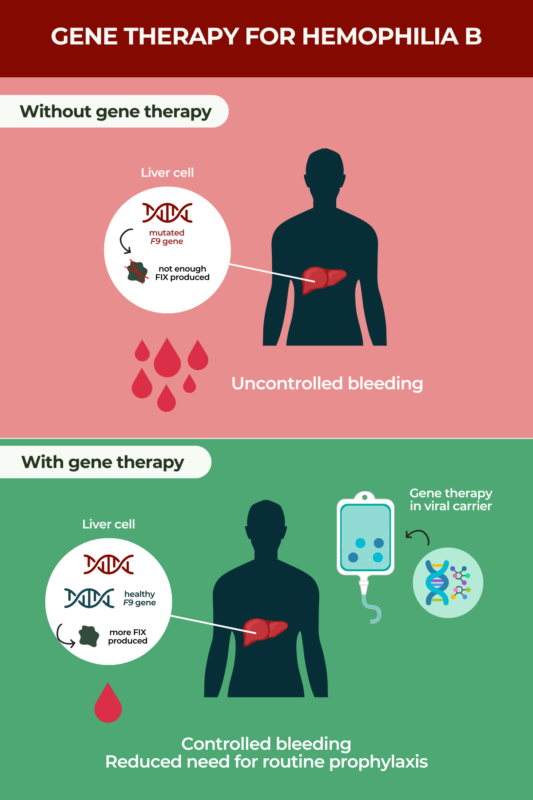
Gene therapy is an approved treatment approach for hemophilia B with potential to be given as a one-time infusion. Hemophilia B is an inherited disease caused by mutations in the F9 gene that interfere with the production of a protein called factor IX (FIX), which is needed for proper blood clotting. As a result, people with hemophilia B are susceptible to uncontrolled or spontaneous bleeding episodes.
Gene therapy directly addresses hemophilia B’s underlying cause by providing a healthy version of F9. While the long-term effects of hemophilia B gene therapy are still being investigated, it is expected to offer lasting bleed control with minimal or possibly no need for regular preventive therapies.
Gene therapy for hemophilia B is currently given to certain men.
What is gene therapy?
In general, gene therapy adds or modifies genetic material as a way to solve the underlying genetic cause of a disease. By doing this, it may be able to relieve symptoms, slow disease progression, or, in some cases, offer a potential cure after one treatment.
When a gene is defective, it can mean that too little or too much of the protein that it encodes is made, or the resulting protein does not work as it should. By correcting or circumventing that genetic mistake, gene therapies can restore a more normal protein production, which can lessen or possibly stop symptoms.
There are two main types of gene therapy.
- Gene replacement therapy, also known as gene transfer or gene addition, is when a new healthy version of a gene replaces the job of the faulty one causing disease. This approach gives the body the blueprint it needs to make its own functional version of that gene’s protein product.
- Gene-editing therapy is when DNA-editing tools can modify a person’s existing DNA. This could include activating helpful genes, inactivating ones that aren’t working properly, correcting a defect in the DNA, or removing problematic DNA. When the gene is corrected, it can then be used as a template for protein production.
It is not easy to get gene therapies inside a person’s cells, where they need to be to work. For this reason, they’re often packaged into a carrier molecule, or vector, that helps them reach the target cells. Viruses are particularly adept at making their way into human cells, so versions of them that don’t cause disease are commonly used as gene therapy vectors.
Once a gene therapy is prepared, it can be delivered to a person’s cells in different ways.
- In vivo, in which the genetic modification happens directly in a person’s body, is when the new genetic material or gene-editing tools are packaged and usually infused into the bloodstream. They’ll be taken up by a person’s cells, where the genetic modification then occurs.
- Ex vivo, in which the genetic modification happens outside the body, is when a person’s cells are isolated from their body, genetically modified in the lab, and then returned to the body.
In either case, once the desired genetic change is in the target cells, the cells’ normal protein-making machinery will be able to repeatedly use that genetic information to produce the correct functioning protein.
How does gene therapy work for hemophilia B?
Gene therapy for hemophilia B works by providing patients with a healthy copy of the F9 gene. When this happens, the body can produce FIX clotting factor, relieving disease symptoms.
It has three important components:
- a healthy, highly functional version of the F9 gene, which is what ultimately enables the body to make its own FIX protein
- a small DNA sequence called a promoter that is attached to the F9 sequence — this promoter specifically causes the gene to be active in liver cells, where most of the body’s blood clotting factors are produced, but not in other cell types where it isn’t needed
- a liver-directed viral vector called an adeno-associated virus (AAV) — AAVs are considered favorable for use in gene therapies because they are very stable and safe, as the virus can invade human cells without causing disease.
Once delivered into the body via a single intravenous (into-the-vein) infusion, the AAV vector will make its way through the bloodstream into the liver, where it will deliver its genetic payload. Liver cells that take up the new gene will now have the tools to make their own FIX protein in the long term.
Prior to receiving hemophilia B gene therapy, patients will have to undergo a number of evaluations to make sure they are eligible to receive treatment, including certain antibody tests and the exclusion of other preexisting conditions.
After receiving gene therapy, patients are monitored for a few hours to make sure they are well enough to go home.
At some point after treatment — if FIX levels are high enough and bleeds are controlled — it might be possible to reduce or stop standard-of-care prophylactic (preventive) hemophilia B therapies.
A similar approach is taken with gene therapy for hemophilia A, which provides liver gene delivery of F8, the gene that is faulty in people with the disease.
Benefits of gene therapy
By addressing the underlying cause of disease, gene therapy can offer a number of benefits for people with hemophilia B. Particularly, with just one infusion, gene therapies have been associated with:
- increased FIX levels
- significantly reduced bleeding episodes
- a reduction or elimination of the need for routine prophylaxis.
Because it should require just one treatment and possibly put an end to needing other types of therapies, gene therapies may help ease a person’s treatment burden and lead to an overall improved quality of life. However, as gene therapies are still new, scientists are continuously learning about their long-term effects. While the benefits are expected to last, it can only be definitively established once treated individuals have been followed for a longer period of time.
Potential risks and side effects
As with any type of hemophilia B treatment, there are potential side effects associated with gene therapy for hemophilia. The specific gene therapy side effects can vary, depending on the particular therapy being used. That said, the most common ones observed in clinical trials have included:
- elevated levels of certain liver enzymes
- headache
- elevated markers indicative of muscle damage
- flu-like symptoms
- infusion-related reactions
- fatigue
- malaise.
More serious risks associated with hemophilia B gene therapy may include:
- severe or life-threatening infusion reactions
- liver toxicity, as liver-directed gene delivery can cause an increase in certain liver enzymes that may require monitoring, treatment, or adjustments to other medications that affect the liver
- liver cancer, as when viral vectors insert themselves into a person’s DNA, it can, in some cases, lead to the uncontrolled cell growth that characterizes cancer. While the AAV vectors used in hemophilia gene therapies generally do not insert into the genome this way, there is a theoretical risk that it could happen.
As with gene therapy benefits, researchers will learn more about the exact side effects and risk profiles of these treatments once more long-term data are available.
Factor replacement therapy vs. gene therapy
Factor replacement therapy, which temporarily provides the body with a working version of FIX, has long been the standard-of-care prophylactic treatment for preventing bleeding episodes in hemophilia B. While both replacement therapy and gene therapy ultimately provide the body with the FIX protein they lack, there is a key difference between them.
Replacement therapies are infused regularly, anywhere from once every couple of weeks to 2-3 times per week depending on the product and a person’s disease severity. In contrast, gene therapy is intended to be given only once in a person’s lifetime.
This difference is due to how the two therapies work. Replacement therapy provides the FIX protein, which will be degraded and needs to be regularly replaced. A gene therapy instead equips the body with the tools it needs for continuous factor IX production.
Is gene therapy covered by medical insurance?
Gene therapies are among the most expensive medications in the world, and whether or not they will be covered by health insurance can depend on the provider and level of coverage. The list price for hemophilia B gene therapy hovers somewhere around $3.5 million.
Many major insurers will provide coverage if a patient meets certain eligibility requirements and the treatment is deemed medically necessary. In most cases, patients will have to go through a pre-approval process prior to treatment for it to be covered. Patients should contact their insurance companies to learn more about their potential coverage.
Other financial assistance programs also may be available for patients who are underinsured or uninsured. A person’s healthcare team may be able to help connect them to potential resources based on their particular situation.
Hemophilia News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
Related articles