Giroctocogene fitelparvovec for hemophilia
Last updated Oct. 30, 2025, by Margarida Maia, PhD

What is giroctocogene fitelparvovec for hemophilia?
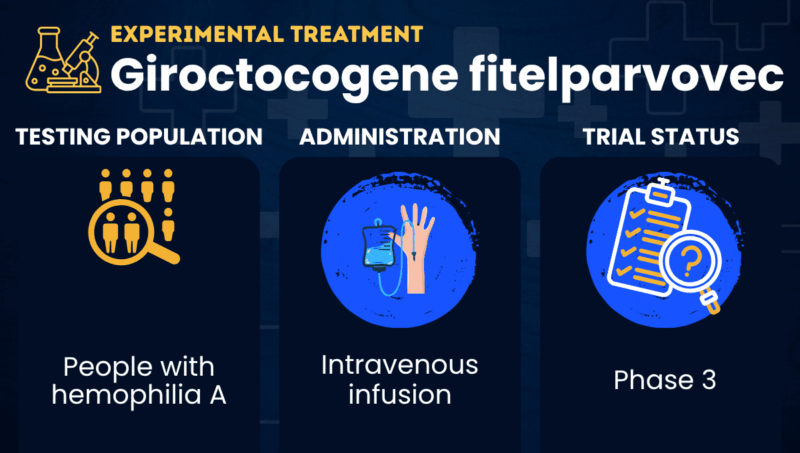
Giroctocogene fitelparvovec, formerly known as SB-525 or PF-07055480, is a gene therapy being developed to reduce the risk of bleeding episodes in people with hemophilia A. It is delivered as a one-time intravenous (into-the-vein) infusion.
Hemophilia A is caused by mutations in the F8 gene, which provides instructions for producing factor VIII (FVIII), a protein needed for the blood to clot. Without it, patients may experience prolonged bleeding episodes that can occur spontaneously or after an injury, and are difficult to control.
Giroctocogene fitelparvovec is designed to deliver a shortened, but functional version of the F8 gene to liver cells using a modified viral vector. Liver cells then use those genetic instructions to produce FVIII, thereby helping to prevent and control bleeding episodes.
Developed by Sangamo Therapeutics in collaboration with Pfizer, the gene therapy has received orphan drug, fast track, and regenerative medicine advanced therapy designations in the U.S., as well as orphan drug status in the European Union. After transitioning the therapy’s Phase 3 clinical development to Pfizer, Sangamo has now regained full rights to giroctocogene fitelparvovec.
Therapy snapshot
| Treatment name: | Giroctocogene fitelparvovec |
| Administration: | Single intravenous infusion |
| Clinical testing: | Currently in Phase 3 testing |
How will giroctocogene fitelparvovec be administered in hemophilia?
In clinical trials involving people with hemophilia A, giroctocogene fitelparvovec has been given as a single intravenous infusion at doses ranging from 9×1011 to 3 x 1013 vector genomes per kilogram of body weight (vg/kg).
Giroctocogene fitelparvovec in hemophilia clinical trials
Giroctocogene fitelparvovec has been tested in two clinical trials involving men with severe hemophilia A. Available results showed the therapy’s ability to increase FVIII levels and reduce the frequency of bleeding episodes.
- Alta (NCT03061201), a Phase 1/2 trial where giroctocogene fitelparvovec was shown to increase FVIII levels to the normal range and reduce annualized bleeding rates in men with severe hemophilia A, particularly at the highest tested dose.
- AFFINE (NCT04370054), a Phase 3 trial where giroctocogene fitelparvovec was shown to reduce annualized bleeding rates in men with moderately severe to severe hemophilia A who had completed at least six months of routine prophylaxis during a lead-in Phase 3 clinical trial (NCT03587116). At least 15 months after the gene therapy’s infusion, the annualized bleeding rate decreased from a mean of 4.7 to 1.2 episodes per year, with most patients (84%) having FVIII levels greater than 5%. After about three years, the annualized bleeding rate decreased even more, to a mean of 0.26 episodes per year, and nearly two-thirds of patients (64%) had not experienced any bleeding episodes.
Giroctocogene fitelparvovec side effects
The most common side effects reported with giroctocogene fitelparvovec during clinical testing include:
- fever
- elevated liver enzymes, a sign the liver may be damaged
- headache.
Hemophilia News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- New test may help fine tune care for those with acquired hemophilia A
- Mild hemophilia patients should monitor their clotting risk after surgery
- Rethinking care expectations in an adult partnership
- Growing up before treatments for hemophilia were safe, part 1
- WHO resolution aims to address gaps in hemophilia care