Adynovate (rurioctocog alfa pegol) for hemophilia
What is Adynovate for hemophilia?
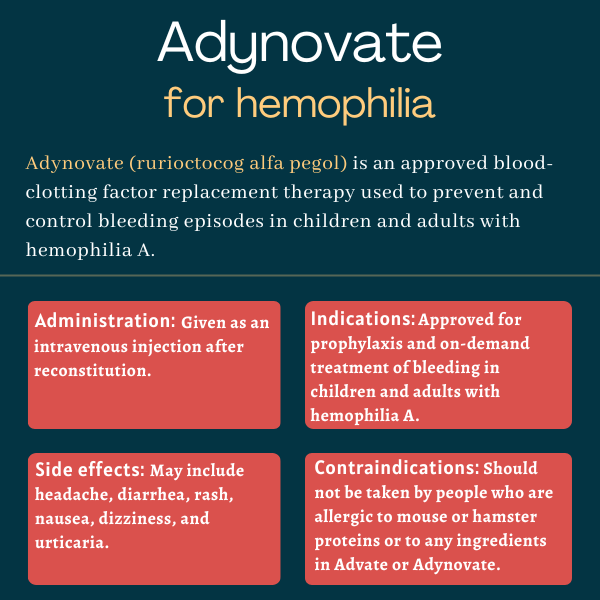
Adynovate (rurioctocog alfa pegol) is a recombinant (lab-made) clotting factor VIII (FVIII) concentrate given as an intravenous (into-the-vein) injection to prevent and control bleeding in children and adults with hemophilia A.
It is indicated for:
- routine prophylaxis (preventive treatment) to reduce the frequency of bleeding episodes
- on-demand treatment to control bleeding episodes
- surgical use to manage bleeding episodes before, during, and after minor or major procedures.
The therapy was developed by Baxter and its spinoff Baxalta, now part of Takeda Pharmaceuticals.
Therapy snapshot
| Brand name: | Adynovate |
| Chemical name: | Rurioctocog alfa pegol |
| Usage: | Prophylaxis and on-demand treatment of bleeding in hemophilia A |
| Administration: | Intravenous injection |
How does Adynovate work?
Hemophilia A is caused by a faulty or missing clotting FVIII, a protein found in the blood. FVIII is needed for blood to form clots and stop bleedings. Without FVIII, people with hemophilia A may experience heavy, longer-than-usual bleeding episodes occurring either spontaneously or as a result of an injury or surgery.
Adynovate is based off Advate (octocog alfa), a recombinant protein that is similar to and works in the same way as the FVIII that is needed for blood to clot normally. It is used as a replacement therapy to temporarily make up for the faulty or missing FVIII, thereby helping to prevent and control bleeding.
The recombinant protein in Adynovate, rurioctocog alfa pegol, is attached to a molecule called polyethylene glycol (PEG) through PEGylation technology licensed from Nektar Therapeutics. This allows the therapy to last longer in the body and to require fewer injections than Advate when used to reduce the frequency of bleeding episodes.
Rurioctocog alfa pegol is made in Chinese hamster ovary cells. These cells were genetically modified in the lab by inserting a gene that provides instructions to make the recombinant protein.
Who can take Adynovate?
The U.S. Food and Drug Administration (FDA) first approved Adynovate in November 2015 for routine prophylaxis and on-demand treatment of bleeding episodes in adults and children, ages 12 and older, with hemophilia A.
This indication was expanded the next year to include its use in children younger than 12, and in surgical settings in both adults and children. Adynovate is approved for the same indication in Canada.
In the European Union, it was approved under the brand name Adynovi in January 2018 for routine prophylaxis and on-demand treatment of bleeding episodes in adults and children, ages 12 and older, with hemophilia A.
Who should not take Adynovate?
Adynovate should not be taken by people who have a known hypersensitivity or allergy to:
- mouse or hamster proteins
- Advate or Adynovate
- any ingredients in Advate or Adynovate, such as mannitol, trehalose, Tris, glutathione, and/or polysorbate 80.
Adynovate is not indicated to treat von Willebrand’s disease, another disease in which blood does not clot properly.
How is Adynovate administered?
Adynovate is given as an infusion directly into the bloodstream. How much is given depends on where the bleeding is located and how heavy it is, as well as on body weight and the severity of hemophilia A.
The concentrate is available as a white to off-white powder supplied in single-use vials containing 250, 500, 750, 1,000, 1,500, 2,000, or 3,000 international units (IU).
It is packaged in a Baxject III reconstitution system with 2 or 5 milliliters (mL) of diluent (sterile water) for injection. Baxject III was approved by the FDA in 2016 for use with Adynovate to allow for an easy reconstitution process of just a few steps. When mixed together, powder and diluent create a clear, colorless solution that is ready to infuse within three hours of reconstitution.
For routine prophylaxis, patients should be given:
- a dose from 40 to 50 IU per kilogram of body weight (IU/kg), twice weekly, in adults and children, ages 12 and older, or
- a dose of 55 IU/kg, twice weekly, in children younger than 12, up to a maximum of 70 IU/kg.
For on-demand treatment and until bleeding has resolved, patients should be given:
- a dose from 10 to 20 IU/kg, given every 12 to 24 hours, in the case of a minor bleeding episode
- a dose from 15 to 30 IU/kg, given every 12 to 24 hours, in the case of a moderate bleeding episode
- a dose from 30 to 50 IU/kg, given every 8 to 24 hours, in the case of a major bleeding episode.
For a minor surgical procedure, such as tooth extraction, a single dose of 30 to 50 IU/kg of Adynovate should be given within one hour before surgery, and then repeated after 24 hours if needed to control bleeding.
For a major surgical procedure, such as a joint replacement, a single dose of 40 to 60 IU/kg should be given within one hour before surgery, and then every 8 to 24 hours (6 to 24 hours for ages younger than 12) to maintain FVIII levels in the desired range until healing is complete.
Each infusion should take no longer than five minutes and can be done at a clinic or at home. But patients should not attempt to inject Adynovate unless they have received proper training from a doctor or other healthcare professional.
A mobile app called myPKFiT is available for use with Adynovate. It allows patients to track treatment, view estimated FVIII levels, and share data with their healthcare provider. Developed by Shire, now part of Takeda, myPKFiT was first CE marked in Europe in 2014 and cleared by the FDA in late 2017 for use with Advate. The mobile app was cleared in late 2020 for use with Adynovate by people with hemophilia A who are 12 years or older and have a body weight of 29 kg (about 64 pounds) or greater.
Adynovate in clinical trials
Adynovate was approved based on the results of clinical trials testing its safety and efficacy in people who previously had been treated for hemophilia A.
Phase 1 trial
A Phase 1 clinical trial (NCT01599819) compared the safety of Adynovate versus Advate in 19 adults, ages 18 to 60, with severe hemophilia A. It also compared the treatments’ pharmacokinetics, or how a medicine moves into, through, and out of the body.
Participants received a single dose of Advate followed by a single dose of Adynovate at either 30 IU/kg or 60 IU/kg. Results showed that the mean half-life of Adynovate — the time taken for its concentration to fall to half its original value — was 1.4 times longer than that of Advate. The mean residence time, or the time it spends in the body, was 1.5 times longer.
Phase 2/3 trial
An open-label pivotal Phase 2/3 clinical trial (NCT01736475), called PROLONG-ATE, compared the safety and efficacy of prophylaxis against on-demand treatment with Adynovate in people previously treated for hemophilia A.
The study included 137 people, ages 12 to 58, with severe hemophilia A. Of this group, 120 received Adynovate as routine prophylaxis (45 IU/kg twice weekly) and 17 as on-demand treatment for bleeding episodes (10 to 60 IU/kg) for six months.
The median annualized bleeding rate, a measure of the number of bleeding episodes adjusted to a one-year time window, was significantly lower for people on routine prophylaxis than for those with on-demand treatment (1.9 vs. 41.5). Of the 101 people who completed routine prophylaxis, 39.6% experienced zero bleeding episodes.
The quality of treatment response was rated using a scale of excellent, good, fair, or none. Among a total of 518 bleeding episodes, 497 (95.9%) were managed with one or two infusions of Adynovate, and 498 (96.1%) were rated as excellent or good in terms of response to treatment. This means that bleeding was controlled after a single infusion (excellent), possibly requiring more than one infusion for complete control (good).
Phase 3 trials
The approval of Adynovate for ages younger than 12 was based on the results of an open-label Phase 3 clinical trial (NCT02210091) testing its safety and efficacy in 66 children previously treated for hemophilia A.
All children, with a mean age of 6 and severe hemophilia A, received Adynovate as routine prophylaxis at a median dose of 51.3 IU/kg twice weekly for six months. During this time, none of the children developed inhibitor (neutralizing) antibodies against FVIII, which may stop Adynovate from working properly.
There were no treatment-related serious side effects, and 25 (38%) children experienced zero bleeding episodes. The median annualized bleeding rate was two, a rate similar to that seen in adults and older children.
Among a total of 70 bleeding episodes, response to treatment was rated as excellent or good for 63 (90%). Most (91.4%) bleeding episodes were managed with one or two infusions of Adynovate.
Another Phase 3 clinical trial (NCT01913405) evaluated the safety and efficacy of Adynovate in 21 people, ages 16 to 61, undergoing surgery. A total of 21 major and five minor surgical procedures were performed. For all 24 surgical procedures with a rating, bleeding control during surgery was rated as excellent. This means that blood loss was less than expected for the type of procedure performed.
Common side effects of Adynovate
The most common side effects that have been reported with Adynovate during clinical trials are:
- headache
- diarrhea
- rash
- nausea
- dizziness
- urticaria (hives).
Hypersensitivity
Hypersensitivity, including anaphylaxis (a severe, whole-body allergic reaction), may occur with Adynovate. If symptoms develop, treatment should be stopped. Urgent medical attention may be required.
Symptoms of an allergic reaction include a rash or hives, itching, swelling, tightness of the throat, chest pain or tightness, difficulty breathing, wheezing, lightheadedness, dizziness, nausea, and vomiting.
Development of inhibitors
The body may form inhibitor antibodies against FVIII. If this happens, it may stop Adynovate from working properly. If the expected levels of FVIII activity are not reached, or if bleeding does not stop with an appropriate dose, a blood test may be done to check for the presence of inhibitor antibodies and measure the amount present.
Use in pregnancy and breastfeeding
Women who are pregnant, planning to become pregnant, or breastfeeding should consult their doctor before taking Adynovate. It is not known if Adynovate is safe to use in pregnancy or if it passes into breast milk or is safe for the breastfed infant.
Hemophilia News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Related articles

 Fact-checked by
Fact-checked by 




