Jivi (damoctocog alfa pegol) for hemophilia
Last updated May 28, 2025, by Lindsey Shapiro, PhD

What is Jivi for hemophilia?
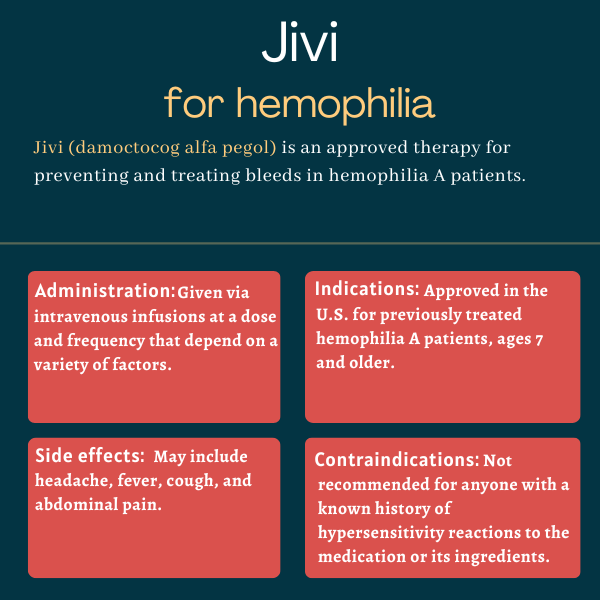
Jivi (damoctocog alfa pegol) is an approved therapy used to prevent and treat bleeds in previously treated adults and children with hemophilia A.
Belonging to a class of standard hemophilia treatments called replacement therapies, Jivi works to prevent and manage bleeding episodes by providing hemophilia A patients with a lab-made version of factor VIII (FVIII), the blood clotting protein they are missing.
Jivi is an extended half-life product, meaning it has modifications that help it last longer in the bloodstream and allow for less frequent dosing compared with standard products. The modification in Jivi is called PEGylation, where the FVIII protein is attached to the nontoxic molecule polyethylene glycol (PEG). This makes FVIII less likely to be broken down and reduces its ability to trigger undesirable immune responses.
Developed by Bayer, Jivi is administered via into-the-vein (intravenous) infusions.
Therapy snapshot
| Brand name: | Jivi |
| Chemical name: | Damoctocog alfa pegol |
| Usage: | Used to prevent and treat bleeds in hemophilia A patients |
| Administration: | Intravenous infusion |
Who can take Jivi?
Jivi is approved in the U.S. for adults and children with hemophilia A, ages 7 and older, who have received previous treatments. For these patients, it is indicated to be used:
- as a routine prophylactic therapy to reduce the frequency of bleeding episodes
- as an on-demand therapy to control active bleeds
- to manage bleeds occurring around the time of surgery.
Jivi is contraindicated for people with a known history of hypersensitivity reactions, including serious allergic reactions, to the medication or any of its ingredients, including PEG and mouse or hamster proteins.
Jivi is not recommended for children younger than 7 due to the increased risk of hypersensitivity reactions and/or loss of treatment efficacy. It is also not indicated for previously untreated patients or people with von Willebrand disease, another bleeding disorder.
Jivi is also approved for similar indications elsewhere, including in the European Union, Japan, and Canada.
How is Jivi administered?
Jivi is given via intravenous infusions that can be self-administered after proper training from a healthcare provider on how to properly store, prepare, and use the medication.
The exact dose and regimen for Jivi is determined by a healthcare provider and depends on factors like the degree of FVIII deficiency, desired levels for bleed control, and a person’s clinical condition.
For routine bleed prevention, Jivi is initially administered twice weekly at an age-dependent dose. The frequency of administration and dose may later be adjusted at a healthcare provider’s discretion based on clinical response.
When used to control active bleeds or for surgical management, the dose and frequency will depend on the severity of the bleed or on how major the surgery is.
Jivi in clinical trials
Jivi’s approval in the U.S. was largely backed by data from three clinical trials involving previously treated adults and children with severe hemophilia A, which demonstrated the therapy’s ability to prevent and control bleeds.
- PROTECT VIII (NCT01580293), where Jivi effectively prevented and controlled active bleeds in adults and adolescents, ages 12-65, with benefits being sustained long-term. Additional analyses involving PROTECT VIII participants showed Jivi also controlled bleeds when used during major surgeries.
- PROTECT VIII Kids (NCT01775618), where Jivi was similarly effective for long-term bleed prevention and control in children ages 2-11.
- Alfa-PROTECT (NCT05147662), where Jivi was tested in children ages 7-11. Pooled data from children ages 7-11 who received Jivi in PROTECT VIII Kids and Alfa-PROTECT showed they had, on average, fewer than two bleeds per year, with about 97% of bleeds that occurred being successfully controlled with one or two Jivi infusions.
Common side effects of Jivi
The most common side effects of Jivi in clinical trials included:
- headache
- fever
- cough
- abdominal pain.
Other less common, but possibly serious side effects could include:
- hypersensitivity reactions, including severe allergic reactions with symptoms like chest or throat tightness, dizziness, low blood pressure, or nausea
- development of inhibitors, a type of neutralizing antibodies against FVIII that can make the treatment less effective
- immune responses against PEG, manifesting as hypersensitivity reactions and/or loss of treatment effectiveness.
Patients will be monitored for clinical or laboratory signs of these complications, and if they arise, treatment may need to be adjusted or discontinued. Hypersensitivity reactions can be a medical emergency, so patients should seek immediate medical care if they arise. They should also let a doctor know if their bleeding is not well controlled on the usual dose of Jivi.
Hemophilia News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- What I’ve learned about obesity, joint pain, and hemophilia bleeds
- FVIII from advanced liver organoids stops bleeding in hemophilia A mice
- When hospitals aren’t prepared for bleeding disorders
- Connection is vital for women in the hemophilia B community
- Patient in Germany gets hemophilia B gene therapy Hemgenix
Related articles