FEIBA (factor eight inhibitor bypassing activity) for hemophilia
What is FEIBA for hemophilia?
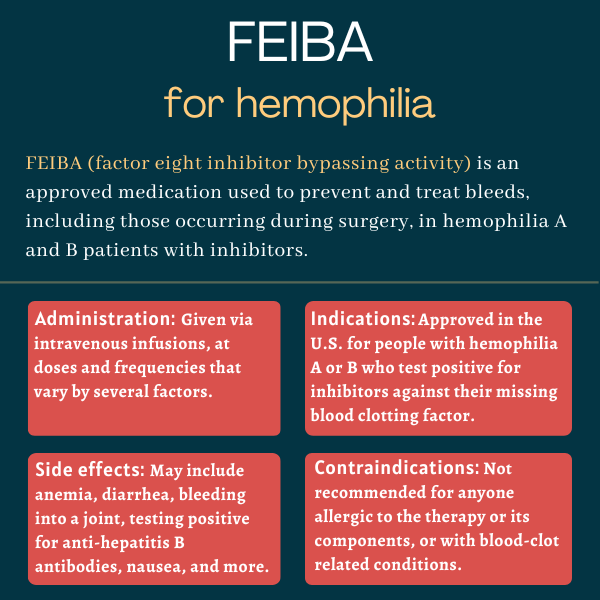
FEIBA (factor eight inhibitor bypassing activity), also known as activated prothrombin complex concentrate and anti-inhibitor coagulant complex, is a bypassing agent that’s approved to prevent or control bleeding episodes in people with hemophilia A and hemophilia B. It can be prescribed for patients with inhibitors, or neutralizing antibodies, against their missing blood clotting factors.
The therapy was originally developed by Baxter Healthcare and marketed by its spinoff Baxalta, which is now part of Takeda. FEIBA is administered via an intravenous or into-the-vein infusion.
Therapy snapshot
| Brand name: | FEIBA |
| Chemical name: | Factor eight inhibitor bypassing activity |
| Usage: | Prevention and treatment of bleeds, including during surgery, in hemophilia A and B patients |
| Administration: | Intravenous infusion |
How does FEIBA work?
Marked by prolonged and excessive bleeding, hemophilia A and B are caused by missing or defective blood clotting factors due to mutations in the genes that code them. A deficiency in factor VIII, or FVIII, is the root cause of hemophilia A, while the lack of factor IX, known as FIX, is associated with hemophilia B.
Replacement therapy, a form of treatment in which patients receive a version of the clotting factor they are missing, is considered a current standard of care for both types of hemophilia. However, as many as 30% of hemophilia A patients and about 5% of those with hemophilia B may develop inhibitors against these clotting factors, rendering replacement therapy ineffective.
As a bypassing agent, FEIBA is designed to effectively circumvent the need for replacement therapy to prevent or control bleeds. It contains several clotting factors that act at multiple sites in the clotting cascade to help restore the production of thrombin, a key protein in the formation of stable blood clots.
As such, the therapy is expected to promote efficient blood clotting in hemophilia A and B patients with inhibitors.
Who can take FEIBA?
In use for on-demand treatment of hemophilia patients with inhibitors since the 1970s, FEIBA was approved in the U.S. for that indication in 1986. Its U.S. label was extended in 2013 to include its use as a routine preventive treatment, or prophylaxis, to prevent or reduce bleeds in these patients. The therapy also is available to prevent bleeds during surgery.
FEIBA is approved in more than 70 countries worldwide and indicated for prophylaxis in more than 60 of them.
Who should not take FEIBA?
FEIBA is contraindicated, or not recommended, for anyone with:
- known allergic reactions to the therapy or any of its components
- disseminated intravascular coagulation, a rare condition characterized by the formation of blood clots throughout the body that block small blood vessels
- sudden formation of blood clots, known as acute thrombosis, or blood vessel blockages, called embolisms, including heart attacks.
The prescribing label for FEIBA has a boxed warning for thrombotic and embolic events, as these have occurred in patients treated with the medication. Patients should be carefully monitored for signs and symptoms of these events.
The therapy also is not indicated for the treatment of bleeding episodes caused by the lack of certain clotting factors in patients who have no inhibitors against FVIII or FIX.
How is FEIBA administered?
FEIBA is given as an infusion directly into the bloodstream. It’s available as a dry powder in single-use color-coded glass vials containing 500 (orange), 1,000 (green), or 2,500 (purple) units per vial. The powder must be diluted in an appropriate liquid solution, which is provided in a separate vial along with a high-flow device and a syringe.
The recommended dose for on-demand treatment to control bleeds is an infusion of 50 to 100 units per kilogram (units/kg) every six to 12 hours, depending on the type of bleeding, and until the bleed or pain — in the case of joint bleeds — is resolved.
Patients undergoing surgery should receive a single dose of 50-100 units/kg before surgery and additional infusions within the same dose range after the procedure, until bleed resolution and healing is achieved. The recommended dose for prophylaxis is 85 units/kg every other day.
Adjustments of doses and administration intervals may be considered for each case based on clinical response. Single doses should not exceed 100 units/kg, and daily doses should not be higher than 200 units/kg. Maximum injection or infusion rate must not exceed 10 units/kg per minute.
FEIBA in clinical trials
The safety and efficacy of FEIBA for on-demand treatment of bleeds were mainly assessed in two prospective clinical trials that followed patients over time.
The first involved 15 hemophilia A patients with inhibitors against FVIII. Each was randomly assigned to receive either FEIBA or a control preparation with a nonactivated prothrombin complex concentrate for treating bleeds. Over 15 months, 150 bleeds, mostly joint and muscle bleeds, were treated.
The results showed a greater proportion of bleeding episodes were resolved with FEIBA relative to the control preparation (64% vs. 52%). The bypassing agent also was found to be significantly superior to the control preparation at stopping bleeds in the same joint or muscle, and at improving joint mobility.
In the second trial, FEIBA was used to treat 165 bleeding episodes in 44 hemophilia A patients with FVIII inhibitors, three hemophilia B patients with FIX inhibitors, and two non-hemophilic patients with acquired FVIII inhibitors. The study demonstrated that more than 90% of the bleeding episodes were controlled with one or more infusions.
The safety and effectiveness of prophylactic treatment with FEIBA were mainly evaluated in two trials: ProFEIBA (NCT00221195) and FEIBA PROOF (NCT00851721).
ProFEIBA trial
The Phase 2/3 ProFEIBA trial evaluated the effects of prophylactic versus on-demand treatment with FEIBA in 34 severe hemophilia A patients, ages 2 and older, with high levels of FVIII inhibitors. Participants received the therapy three times a week for the first six months as a prophylaxis regimen, followed by a three-month treatment-free period and a subsequent six-month period of on-demand treatment.
The prophylaxis regimen was generally safe, and was found to reduce both bleeding episodes and target-joint bleeding compared with the on-demand regimen, the results showed. Bleeding episodes were reduced by 62% and target-joint bleeding by 72%.
FEIBA PROOF trial
The global Phase 3 FEIBA PROOF study compared prophylactic treatment using the medication with on-demand use in hemophilia A and B patients, ages 4 to 65, with inhibitors. A total of 17 patients were given the therapy as prophylaxis, while 19 were treated on-demand over a year.
Prophylactic treatment was associated with a significant 72.5% drop in the median annualized bleeding rate relative to the on-demand regimen. Three patients on prophylaxis experienced no bleeding episodes, while none in the on-demand group did. Also, the total use of FEIBA was significantly higher in the on-demand group.
The rates of treatment-related adverse events were comparable between the two regimens.
FEIBA STAR trial
A Europe-based Phase 3b/4 study, called FEIBA STAR (NCT02764489), assessed the safety and tolerability of FEIBA when reconstituted in a regular versus 50% reduced volume, and given at faster infusion rates in 33 men with hemophilia A or B with inhibitors.
The rates of relevant adverse events were comparable between regular versus reduced volume doses, according to the results. Also, administering a reduced volume at faster rates had a similar safety and tolerability profile to the approved regimen. These findings support the potential use of FEIBA at a 50% reduced volume and administered at a faster infusion rate to help reduce treatment burden.
Phase 4 trial
Potential safety concerns were raised regarding combined treatment with FEIBA and the approved therapy Hemlibra (emicizumab). Thus, a Phase 4 trial (NCT04205175) investigated FEIBA’s safest dose range in severe hemophilia A male patients with inhibitors who had been on prophylactic Hemlibra for at least two months. Hemlibra is a hemophilia A approved therapy that mimics the activity of FVIII.
The results suggested that a single infusion of FEIBA, at a dose of up to at least 75 units/kg is safe and effective to treat breakthrough bleeds for most patients on Hemlibra without thrombotic risk factors. FEIBA doses lower than those recommended in the label may not be effective for treating breakthrough bleeds in these patients, the study also indicated.
Common side effects of FEIBA
The most common side effects associated with FEIBA are:
- anemia, or low red blood cell counts
- diarrhea
- bleeding into a joint
- testing positive for hepatitis B surface antibody
- nausea
- vomiting.
Serious side effects of FEIBA include allergic reactions and thromboembolic events, which occur when a blood clot breaks off, travels through the body, and blocks another blood vessel.
Thromboembolic events
Thromboembolic events, such as blood vessel blockage in the lungs, stroke, heart attack, and deep vein blood clots, can occur with FEIBA, particularly after high doses have been administered (above 200 units/kg per day) and/or in patients with risk factors for blood clots.
These events also may occur with FEIBA when given along with antifibrinolytics that may prevent blood clot breakdown, such as tranexamic acid and aminocaproic acid. Such therapies should not be used within about six to 12 hours after a FEIBA infusion.
Cases of thrombotic microangiopathy (TMA) were reported in a clinical trial in which patients received on-demand treatment with FEIBA while also on prophylaxis with Hemlibra. TMA is a rare condition marked by red blood cell destruction, low platelet counts, and the formation of blood clots in small blood vessels that can damage internal organs, particularly the kidneys.
As such, the potential benefits of FEIBA should be weighed against the potential risk of these thromboembolic events in patients with thrombotic risk factors and those taking certain medications.
These patients, along with any individual treated with more than 100 units/kg of FEIBA, should be monitored for signs and symptoms of blood clot-related events. If clinical signs or symptoms occur — such as chest pain or pressure, shortness of breath, altered consciousness, vision or speech problems, limb or abdomen swelling, and/or pain, patients should seek immediate medical attention and discontinue treatment.
Allergic reactions
Allergic reactions, which can include hives, chest tightness, wheezing, difficulty breathing, low blood pressure, gastrointestinal manifestations, and/or swelling of the deeper layers of the skin or mucous membranes, have been reported with FEIBA. Other infusion reactions, such as chills, fever, and high blood pressure, also have been reported.
If a severe allergic reaction is suspected, the medication should be immediately discontinued and the patient should be given appropriate treatment to manage it.
Transmission of infectious agents
FEIBA is made from human blood and, despite several safety and preventive measures being implemented in its manufacturing process, it may still carry a risk of transmitting infectious agents, such as viruses, to patients.
Abnormal blood tests
Given that the therapy contains isohemagglutinins — naturally occurring antibodies against red blood cells from other people, but not their own — patients on FEIBA may wrongly test positive in assays assessing for the presence of self-reactive antibodies against red blood cells.
Use in pregnancy and breastfeeding
No animal or human studies to date have evaluated whether FEIBA can cause harm to a developing fetus or affect a person’s reproductive ability. Likewise, it is not known if it can pass into breast milk. Patients should talk with their healthcare professionals about using FEIBA during pregnancy and breastfeeding to see if the benefits justify potential risks.
Hemophilia News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
Related articles

 Fact-checked by
Fact-checked by 




