NovoSeven RT (eptacog alfa) for hemophilia
What is NovoSeven RT for hemophilia?
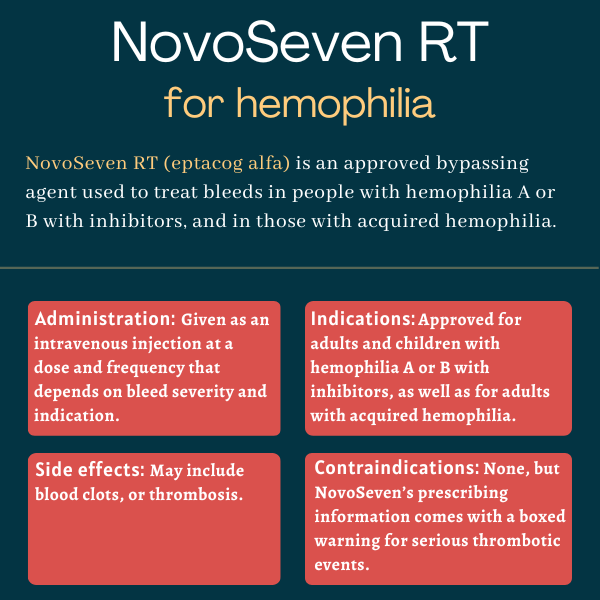
NovoSeven (eptacog alfa [activated]) is a bypassing agent that’s approved to treat bleeding episodes, including those occurring during surgery, in adults and children with hemophilia A or B who have developed inhibitors — neutralizing antibodies against certain clotting factors.
It’s also approved for adults with acquired hemophilia, for the treatment of bleeding episodes and the prevention of bleeds in surgical interventions or invasive procedures.
The medication initially was developed by Novo Nordisk as NovoSeven, but now is sold in a formulation, called NovoSeven RT, that’s designed to be more stable at room temperature. It is administered via an intravenous or into-the-vein injection.
In addition to hemophilia indications, NovoSeven RT is approved for individuals with congenital FVII deficiency and Glanzmann’s thrombasthenia that is unresponsive to platelet transfusions.
Therapy snapshot
| Brand name: | NovoSeven RT |
| Chemical name: | Eptacog alfa (activated) |
| Usage: | Treatment and control of bleeding episodes in patients with hemophilia A or B, or acquired hemophilia |
| Administration: | Intravenous injection |
How does NovoSeven RT work?
Hemophilia is caused by the lack or malfunction of certain proteins involved in blood clotting, which lead to episodes of uncontrolled bleeding.
After a blood vessel injury, one of these clotting factors, called factor VIIa (FVIIa), is normally activated and kicks off a chain of reactions that ultimately produce fibrin — the protein that sticks together at the site of injury and forms a clot.
NovoSeven RT is a recombinant, or lab-made, form of FVIIa that is similar to the one naturally produced in the body. When infused into the bloodstream during a bleeding episode, it stimulates the body’s natural blood clotting cascade.
The mechanism by which NovoSeven RT promotes blood clotting bypasses the need for factor VIII (FVIII) or factor IX (FIX) — the clotting factors that are missing or being targeted by inhibitors in patients with hemophilia A or B. For this reason, NovoSeven RT can be used to control bleeds in hemophilia patients with inhibitors, for whom conventional replacement therapies may no longer be effective.
The older NovoSeven worked via the same mechanism, but the newer formulation has been modified to be more stable at room temperature — hence the RT.
Who with hemophilia can take NovoSeven RT?
The original NovoSeven formulation was first approved by the U.S. Food and Drug Administration (FDA) in March 1999 for the treatment of bleeding episodes in adults and children with hemophilia A or B with inhibitors. It became the first recombinant bypassing agent to be approved in the U.S. for the treatment of hemophilia patients with inhibitors.
That approval was expanded in October 2006 to include adults with acquired hemophilia.
In May 2008, the NovoSeven RT formulation was cleared for the same indications.
To date, NovoSeven RT is the only FDA-approved bypassing agent that’s available to treat hemophilia patients of all ages with inhibitors, as well as people with acquired hemophilia.
NovoSeven also is approved for similar indications in the European Union, where the therapy first gained approval in February 1996.
Who should not take NovoSeven RT?
There are no contraindications for NovoSeven’s use.
However, the therapy’s prescribing information comes with a boxed warning noting that NovoSeven may cause serious blood clot-related, or thrombotic, events. For this reason, patients on NovoSeven RT should be closely monitored for signs or symptoms of a thrombotic event. If such an event occurs, NovoSeven’s dose should be reduced or treatment should be discontinued altogether, depending on the patient’s condition.
How is NovoSeven RT administered in hemophilia?
NovoSeven RT comes as a white powder in single-dose vials containing 1, 2, 5, or 8 mg of recombinant FVIIa and a separate solvent. Before use, the powder is dissolved in the solvent at room temperature to produce a clear, colorless solution at a concentration of 1 mg FVIIa per 1 mL of liquid.
The solution is administered via an intravenous injection, a process that usually takes 2-5 minutes to complete and that must be carried out within three hours of reconstitution.
NovoSeven RT usually is given by healthcare professionals with appropriate experience. In some cases, patients or caregivers may be able to administer NovoSeven RT after receiving appropriate training.
During an acute bleeding episode, NovoSeven RT should be administered at doses that will depend on the indication and severity of bleeds:
- For hemophilia A or B patients with inhibitors, a dose of 90 micrograms per kilogram of body weight (mcg/kg) should be given every two hours until bleeding stops. For severe bleeds, administration is continued every 3-6 hours after bleeding is controlled.
- For acquired hemophilia patients, a dose of 70-90 mcg/kg should be administered every two to three hours until bleeding stops.
For managing bleeds during surgery, NovoSeven RT should be administered at the following doses:
- For those with hemophilia A or B with inhibitors, a dose of 90 mcg/kg should be given immediately before surgery, and every two hours during surgery. After a minor surgery, the medication should also be given at the same dose, every two hours for two days, then every 2-6 hours until healing occurs. After a major surgery, the medication should be administered at a dose of 90 mcg/kg every two hours for five days, then every four hours or via continuous infusion at a rate of 50 mcg/kg per hour until healing occurs.
- For individuals with acquired hemophilia, a dose of 70-90 mcg/kg should be administered immediately before surgery. and every 2-3 hours for the duration of surgery and until bleeding stops.
The doses and frequency of administration may be adjusted by a healthcare provider based on bleeding severity.
NovoSeven RT in hemophilia clinical trials
Many clinical trials have been conducted since the 1990s that have established the safety and efficacy of NovoSeven at various doses in adults and children. Trials also were conducted for diverse applications of the therapy, including surgical management.
Trials for hemophilia A and B with inhibitors
A double-blind randomized trial, conducted in the late 1990s, compared two doses of NovoSeven — 35 mcg/kg or 70 mcg/kg — for the treatment of bleeding events in hemophilia patients. That study involved 78 people with hemophilia A and B, both with and without inhibitors. As the trial was double-blind, neither participants nor researchers knew which patients were given which dose.
Patients received an initial NovoSeven infusion at a treatment center within 4-18 hours after a bleed began; that dose was repeated every 2.5 to four hours. The effects of treatment were evaluated after 12 hours or when treatment stopped, whichever occurred first.
The effects of the 35 mcg/kg dose were deemed excellent in 59% of the cases and effective in 12%. The 70 mcg/kg dose was found to be excellent in 60% of the cases and effective in 11%. The average number of injections needed to stop bleeding was 2.8 for the 35 mcg/kg dose, and 3.2 for the 70 mcg/kg dose.
Two additional clinical trials were conducted to evaluate NovoSeven’s safety and efficacy during or after surgery in hemophilia A or B patients with inhibitors.
One trial evaluated two doses of NovoSeven — this time, 35 mcg/kg or 90 mcg/kg — in 29 patients undergoing major or minor surgical procedures. Participants received their assigned dose once prior to surgery, during surgery as needed, and every two hours for two days after surgery. Additional doses were administered every 2-6 hours for an additional three days, as needed.
This study also was randomized and double-blind.
The results showed that bleeding was controlled during surgery in 28 patients. Two days after surgery, bleeding control was deemed satisfactory in all 14 patients treated with the 90 mcg/kg dose and in 11 of the 15 treated with the 35 mcg/kg dose.
A second open-label trial compared the safety and efficacy of a single dose of NovoSeven versus a continuous infusion in 24 patients undergoing major surgery. Because this trial was open-label, the participants and researchers knew which patients were receiving each dose.
The two approaches had comparable efficacy in achieving and maintaining bleeding control through 10 days, the results showed.
Trials for acquired hemophilia
Data supporting NovoSeven’s use in acquired hemophilia came from four studies involving patients who received the medication through compassionate use programs conducted by Novo Nordisk, as well as those included in the Hemophilia and Thrombosis Research Society (HTRS) registry.
The analyses overall involved 70 patients who received NovoSeven for 113 bleeding episodes, surgeries, or traumatic injuries. Doses of NovoSeven ranged from 31 to 197 mcg/kg, with a mean dose of 90 mcg/kg and a mean number of injections of six per day.
The overall efficacy across all patients was 78%. Importantly, the therapy was more effective when used as a first-line therapy (86%) than in people who had received prior treatment (70%).
Common side effects of NovoSeven RT
The most common side effects experienced by patients using NovoSeven in clinical trials include:
- blood clots, or thrombotic events.
Blood clotting
NovoSeven’s label comes with a boxed warning noting that serious thrombotic events, or blood clotting issues, may occur in patients given the therapy. Individuals using other clotting medications, older individuals, and those with a history of cardiovascular disease may be at an increased risk of blood clots.
Prior to receiving NovoSeven RT, all patients should discuss with their healthcare providers the signs and symptoms associated with thrombotic events. Additionally, all patients should be monitored for the development of blood clots.
If a blood clot event occurs, the dose of NovoSeven RT should be lowered or stopped at a healthcare provider’s discretion.
Allergic reactions
Allergic reactions, including a potentially life-threatening reaction known as anaphylaxis, can occur with NovoSeven RT. Patients with a known sensitivity to mouse, hamster, or cow proteins may be at a higher risk of experiencing allergic reactions.
If an allergic reaction occurs, NovoSeven should be discontinued and patients should receive appropriate treatment.
Use in pregnancy and breastfeeding
There are no well-controlled studies evaluating the use of NovoSeven RT in women during pregnancy or breastfeeding. However, the therapy was associated with increased mortality in animal reproductive studies.
Patients who are pregnant or wish to become pregnant, or who are breastfeeding or plan to breastfeed while using NovoSeven should discuss this with their healthcare provider.
Hemophilia News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- When scars from my IV infusions lead to false assumptions
- Blood immune markers may predict ITI response in hemophilia A
- Going back to basics and finding strength on the floor
- Hympavzi lowers bleeding rates for hemophilia patients with inhibitors
- How our oldest son and hemophilia entered our lives, part 2
Related articles

 Fact-checked by
Fact-checked by 



