Nuwiq (simoctocog alfa) for hemophilia
What is Nuwiq for hemophilia?
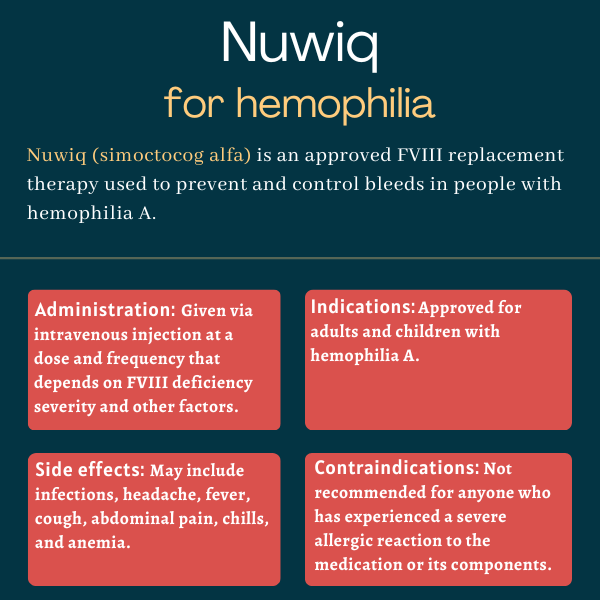
Nuwiq (simoctocog alfa) is an approved therapy that works to prevent and control bleeding episodes, including those occurring during surgery, in adults and children with hemophilia A.
The medication, marketed by Octapharma, is administered via an intravenous, or into-the-vein, injection.
Therapy snapshot
| Brand name: | Nuwiq |
| Chemical name: | Simoctocog alfa |
| Usage: | Used to prevent and treat bleeds, including those occurring during surgery, in hemophilia A patients |
| Administration: | Intravenous injection |
How does Nuwiq work?
Hemophilia A is caused by a deficiency in a clotting protein called factor VIII (FVIII). Without a working version of this protein, blood cannot clot properly, leading to an increased risk of bleeding.
Nuwiq contains a recombinant, or lab-made, version of FVIII that can be administered to replace the protein that’s defective or missing in people with hemophilia A. The therapy is made from a human cell line and does not contain any chemical modifications or protein fusions; it is essentially identical to the active form of human FVIII.
Other clotting factor replacement therapies often contain such modifications, which are made with the goal of improving the medication’s stability in the body, so they don’t need to be administered as often. However, these changes can increase the likelihood that the body’s immune system will see the therapy as a threat and launch an attack that renders the treatment ineffective.
Who can take Nuwiq?
Nuwiq was approved by the U.S. Food and Drug Administration (FDA) in September 2015 to treat children and adults with hemophilia A. That decision made Nuwiq the first recombinant FVIII product produced in a human cell line without chemical modifications or protein fusions to be approved for hemophilia A in the U.S.
The therapy is specifically indicated to be used as a routine prophylactic or preventive treatment to lower the frequency of bleeding episodes. It also can be prescribed as an on-demand treatment to control bleeds, and to manage any that occur during surgery (perioperative management).
Nuwiq also is approved for similar indications in the European Union, and in Australia and several other countries.
Who should not take Nuwiq?
Nuwiq is contraindicated, or not recommended, to anyone with a history of severe allergic reactions to the medication or any of its components. It’s also not indicated for treating von Willebrand disease, another inherited bleeding disorder.
How is Nuwiq administered?
Nuwiq is given via an injection into the bloodstream, known as intravenous or into-the-vein administration, and may be self-administered after proper training.
The therapy is available as a white powder that comes in single-use vials containing 250, 500, 1000, 1,500, 2,000, 2,500, 3,000, or 4,000 international units (IUs) of FVIII. The powder must be reconstituted, or diluted, in 2.5 mL of sterile water up to three hours before being administered. Pre-filled syringes of sterile water are provided.
The treatment dosage and duration depend on the severity of the FVIII deficiency, the location and extent of the bleed, and the patient’s clinical condition and response. The rate of administration should be determined by the patient’s comfort level, up to a maximum of 4 mL/min.
For routine prophylaxis, the recommended dose of Nuwiq for adults and adolescents ages 12 and older is 30-40 IU per kilogram of body weight every other day. For children ages 2 through 11, the recommended dose is 30-50 IU/kg every other day, or three times per week. For those younger than 2, the recommended dose is 20-50 IU/kg once per week to every other day. Dosing schedules can be further adjusted at the discretion of the treating physician.
For on-demand treatment, the dose of Nuwiq is calculated based on the severity of the bleed.
- For minor bleeds, such as superficial muscle or soft tissue and oral bleeds, Nuwiq should be given every 12 to 24 hours to maintain FVIII activity levels at 20%-40% for at least one day until the bleed is resolved.
- For moderate to major bleeds, such as joint bleeds or those occurring after trauma, Nuwiq should be given every 12 to 24 hours to maintain FVIII activity levels at 30%-60% for three to four days, or more, until the bleed is resolved.
- For life-threatening bleeds, including those occurring in the brain, gastrointestinal tract, or resulting from fractures or head trauma, Nuwiq should be given every eight to 24 hours to maintain FVIII activity levels at 60%-100% until the bleed is resolved.
When used to manage bleeds occurring during surgery, the dose of Nuwiq is adjusted based on the scale of the operation.
In patients undergoing minor surgery, such as tooth extraction, Nuwiq should be given every 24 hours to maintain FVIII activity levels at 30%-60% for at least one day until healing is achieved. In patients undergoing major surgery, such as joint replacement procedures, Nuwiq should be given every eight to 24 hours to maintain FVIII activity levels at 80%-100% until wound healing is complete. Also, Nuwiq should be continued for at least another seven days in patients having major surgery to maintain FVIII activity levels at 30%-60%.
Patients should not self-administer Nuwiq until a healthcare provider has shown them how to correctly mix and give themselves the treatment. All individuals self-administering Nuwiq should carefully follow their doctor’s instructions, and in case of doubt, contact their healthcare provider before using the medication.
Additional instructions on how to use Nuwiq were issued in September 2020.
Nuwiq in clinical trials
The efficacy of Nuwiq was mainly evaluated in three open-label clinical trials: GENA-01 (NCT00989196), GENA-08 (NCT01125813), and GENA-03 (EudraCT2010-018644-14). Each tested the therapy in previously treated patients with severe hemophilia A.
Two of the studies assessed the efficacy of Nuwiq prophylaxis — GENA-08 in 32 adults and GENA-03 in 59 children ages 2-12. Both of these studies also evaluated the efficacy of Nuwiq as an on-demand treatment whenever bleeds occurred.
GENA-01, meanwhile, assessed the efficacy and pharmacological properties of Nuwiq as an on-demand treatment in 20 adults and two adolescents.
Bleed control
Across these three studies, a total of 1,124 bleeding episodes in 69 patients were treated with Nuwiq. Most of these episodes — 986 in total — occurred in GENA-01, where patients weren’t receiving preventive treatment.
The efficacy of Nuwiq in controlling these bleeding episodes was rated as “excellent” or “good” in 94% of the cases, meaning there was clear pain relief and/or bleed control within 8-12 hours after dosing, and no more than two injections were required to control bleeding. The remaining 6% of bleeds were classified as moderate, meaning improvements were seen within 12 hours, but more than two injections were required to totally control bleeds. Most bleeding episodes (90%) required only one injection of Nuwiq to be controlled.
During the three studies, Nuwiq was used to control surgery-related bleeds in 28 major surgical procedures. The therapy’s effectiveness at controlling bleeds in major surgeries was rated as “excellent” in 82%, as “good” in 14%, and as “moderate” in just one case. In minor surgeries, the therapy’s efficacy at controlling bleeds was considered to be “excellent” in all cases.
Prophylaxis
In GENA-08, Nuwiq was given every other day at a dose of 30-40 IU/kg for at least six months, while in GENA-03 the therapy was administered either every other day or three times weekly for at least six months.
Data from the two prophylaxis trials showed that, after six months on preventive Nuwiq, the median annualized bleed rate was 0.9 bleeds per year in adults participating in GENA-08 and 1.9 bleeds per year in children enrolled in GENA-03. Compared with previous on-demand treatment with another FVIII product, bleed rates dropped by 96% in adults in GENA-08 and by 93% in children in GENA-03.
In both adults and children, the median number of spontaneous bleeds was zero, meaning most patients didn’t have any spontaneous bleeds while on Nuwiq prophylaxis. Most bleeds that did occur were not major, and none were life-threatening.
A total of 49 children who completed GENA-03 opted to enroll in a long-term extension study, called GENA-13 (EudraCT2011-001785-17), to continue prophylactic treatment with Nuwiq for an additional mean period of 2.5 years. Over the course of the extension study, the median annualized bleed rate was 1.72, with nearly half of the children (45%) remaining free of spontaneous bleeds.
Safety
In clinical trials involving previously treated people with hemophilia A, there were no reports of patients developing neutralizing antibodies, known as inhibitors, against FVIII after switching to Nuwiq. Additionally, none of the patients experienced a serious allergic reaction, discontinued treatment due to side effects, or died.
A Phase 3 clinical trial called NuProtect (NCT01712438) evaluated Nuwiq’s immunogenicity, or ability to trigger the development of FVIII inhibitors, in previously untreated patients with severe hemophilia A.
Final data from NuProtect showed that 28 of 105 evaluable patients — 26.7% in total — developed FVIII inhibitors, with 17 (16.2%) having high inhibitor levels. Nevertheless, the rate of inhibitor development among previously untreated patients with hemophilia A treated with Nuwiq was lower than that seen in patients treated with FVIII products derived from hamster cell lines in other studies.
Common side effects of Nuwiq
The most common side effects of Nuwiq reported in clinical trials include:
- upper or lower respiratory tract infections
- headache
- fever
- cough
- inflammation in the nose or throat
- chills
- abdominal pain
- joint pain
- anemia.
Allergic reactions
Allergic reactions, including severe reactions (anaphylaxis), are possible in patients treated with Nuwiq. Potential early signs of an allergic reaction may include swelling, chest tightness, shortness of breath, and wheezing.
If a patient experiences an allergic reaction to the therapy, appropriate treatment to manage the reaction should be given at once, and Nuwiq should be discontinued.
Development of inhibitors
In some cases, the immune system may mistake replacement therapies like Nuwiq for an infectious threat, causing immune cells to make antibodies against the therapy. These antibodies are commonly called inhibitors because they can stop the medication from being effective.
Patients on Nuwiq should be regularly monitored for the presence of inhibitors, especially if they aren’t responding to treatment as expected. FVIII activity levels in the blood also should be monitored in patients on Nuwiq to ensure that adequate levels can be achieved and maintained.
Use in pregnancy and breastfeeding
There are no adequate studies on the use of Nuwiq in people who are pregnant or breastfeeding. Moreover, no animal studies have evaluated the therapy’s effects on reproduction or fetal development, so it is unclear whether Nuwiq can affect reproduction or cause harm to a developing fetus.
It’s recommended that the therapy should only be given to pregnant patients if clearly needed, and its use while breastfeeding should be carefully weighed against the potential risks for the nursing infant.
Hemophilia News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Packing for a move conjures an unwelcome guest: Joint pain
- When scars from my IV infusions lead to false assumptions
- Blood immune markers may predict ITI response in hemophilia A
- Going back to basics and finding strength on the floor
- Hympavzi lowers bleeding rates for hemophilia patients with inhibitors

 Fact-checked by
Fact-checked by 




