Obizur (susoctocog alfa) for hemophilia
What is Obizur for hemophilia?
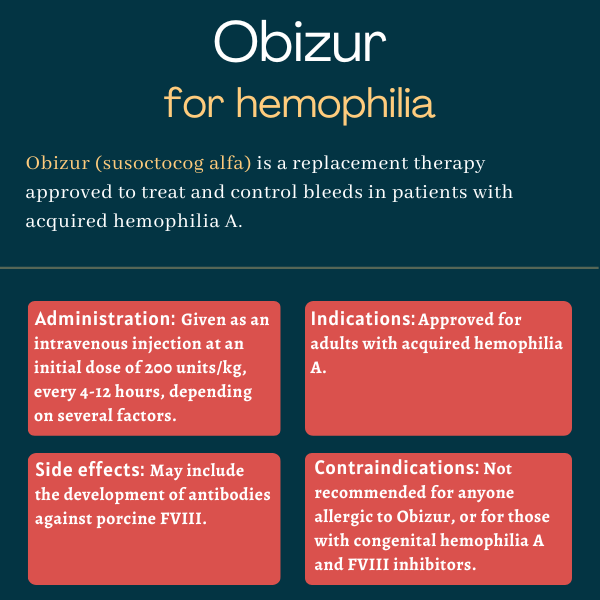
Obizur (susoctocog alfa) is a recombinant, or man-made, antihemophilic factor therapy approved for the on-demand treatment of bleeding episodes in adults with acquired hemophilia A.
The therapy was developed by Baxter and its spinoff Baxalta, now part of Takeda Pharmaceuticals. It is given as an intravenous (into-the-vein) injection.
Therapy snapshot
| Brand name: | Obizur |
| Chemical name: | Susoctocog alfa |
| Usage: | On-demand treatment of bleeding episodes in acquired hemophilia A patients |
| Administration: | Intravenous injection |
How does Obizur work?
Acquired hemophilia A occurs when the body mistakes the clotting factor VIII (FVIII) as foreign and develops self-reactive antibodies against it. FVIII is a protein needed for blood to clot normally.
These self-reactive antibodies inactivate the protein, preventing it from taking part in the normal blood clotting process. As a result, people with acquired hemophilia A can experience excessive, uncontrolled bleeding episodes.
Obizur contains a protein called susoctocog alfa, a modified porcine (pig-derived) version of FVIII that works in the same way as human FVIII. But it takes on a slightly different shape, so it is not easily targeted by self-reactive antibodies. As such, it can be used as a replacement therapy to temporarily supply the FVIII that’s been inactivated and help to control bleeding.
Susoctocog alfa is produced in baby hamster kidney cells, which have been modified to carry a gene that provides instructions to make the protein.
Who can take Obizur?
The U.S. Food and Drug Administration (FDA) approved Obizur in October 2014 for the on-demand treatment of bleeding episodes in adults with acquired hemophilia A, making it the first recombinant porcine FVIII treatment to be approved for that indication in the country.
The therapy was approved the following year for the same indication in Canada and the European Union.
Who should not take Obizur?
Obizur is contraindicated, or not recommended, for patients who:
- have had life-threatening allergic reactions to the treatment, any of its components, or to hamster proteins
- have congenital hemophilia A and neutralizing antibodies against FVIII (inhibitors), due to the risk of an anamnestic reaction — a rapid, heightened production of antibodies following re-exposure — to human or porcine FVIII.
The medication also is not indicated for the treatment of von Willebrand disease, another disease in which the blood does not clot properly.
How is Obizur administered?
Obizur is given as a slow injection directly into the bloodstream. The dose, and how often it is administered, depends on the patient’s clinical condition, how well they respond to treatment, target FVIII levels, and the location and severity of the bleed.
The therapy is available as a white powder, supplied in single-use vials containing 500 FVIII units. It needs to be reconstituted, or diluted, using the 1 mL of sterile water provided in pre-filled syringes. The final solution should be administered within three hours of reconstitution at a rate of 1 to 2 mL per minute.
The recommended initial dose of Obizur is 200 units per kilogram (kg) of body weight, given every 4-12 hours depending on a patient’s clinical response and the measured FVIII levels.
For minor and moderate bleeding episodes, such as superficial muscle bleeds, patients should reach target FVIII levels in the range of 50-100 units per deciliter (U/dL).
For major bleeding episodes, such as moderate to severe muscle bleeds and bleeds occurring in the brain or gastrointestinal tract, patients should reach target FVIII levels in the range of 100-200 U/dL to treat acute bleeding episodes, and 50-100 U/dL with continued treatment as needed, once bleeding is under control.
Patients should be monitored for FVIII activity 30 minutes and again three hours after receiving the initial dose, and 30 minutes after being given subsequent doses.
Treatment with Obizur should be carried out under the supervision of a doctor with experience in treating hemophilia.
Obizur in clinical trials
Obizur was approved based on data from an open-label Phase 2/3 clinical trial (NCT01178294) designed to test the treatment’s safety and efficacy in 29 adults with acquired hemophilia A who were experiencing a serious bleeding episode.
Patients were given Obizur at an initial dose of 200 units/kg. Additional doses depended on how well patients responded to treatment and measured FVIII levels.
Within 24 hours, all 28 patients who were examined for treatment efficacy showed a positive response to Obizur, which was the trial’s main goal. The response to Obizur was considered positive if bleeding had stopped or was significantly reduced.
That positive response was observed within eight hours in most (19 patients, or 67.9%), with the others responding positively over the following 16 hours. In 24 patients (85.7%), bleeding eventually stopped completely — but better responses were observed in patients who received Obizur as a first-line treatment compared with those who first had been given other therapies (94% vs. 73%). No serious side effects were reported.
The median dose per infusion to successfully treat bleeding episodes was 133 U/kg; the median total dose was 1,523 U/kg.
In the initial 24-hour period, a median of three infusions were given at a median dose of 200 U/kg. When treatment was required beyond 24 hours, a median of 10.5 infusions at a median dose of 100 U/kg were given for a median of six days to control bleeding.
Common side effects of Obizur
The most common side effect associated with Obizur in clinical trials was the development of antibodies against porcine FVIII.
Allergic reactions
Allergic reactions, including a potentially life-threatening allergic reaction called anaphylaxis, may occur with Obizur. Early signs of an allergic reaction can include tissue swelling, chest tightness, shortness of breath, low blood pressure, wheezing, hives, and severe itching of the skin. If these symptoms arise, Obizur should be stopped and appropriate treatment initiated.
Development of antibodies
Some patients treated with Obizur developed antibodies against porcine FVIII, which can render the therapy less effective. In such cases, other treatment options may be considered, including the initiation of treatment with bypassing agents.
If the expected levels of FVIII are not reached, or if bleeding does not stop with an appropriate dose, a blood test should be done to check for the presence of anti-porcine FVIII antibodies. It is also recommended to check for the presence of these antibodies prior to starting treatment with Obizur.
Use in pregnancy and breastfeeding
It is not known if Obizur can be harmful to a developing fetus, if it passes into breast milk, or if it is safe for the breastfed infant. Women who are pregnant or breastfeeding, or who plan to become pregnant or breastfeed, should consult their doctor before taking Obizur.
Hemophilia News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Going back to basics and finding strength on the floor
- Hympavzi lowers bleeding rates for hemophilia patients with inhibitors
- How our oldest son and hemophilia entered our lives, part 2
- Tips for supporting and validating loved ones with hemophilia
- Building and nurturing resilience in children with hemophilia
Related articles

 Fact-checked by
Fact-checked by 


